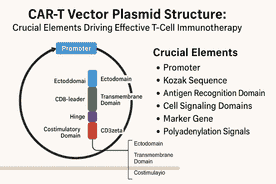
Chimeric antigen receptor (CAR) T-cell remedy has revolutionized most cancers therapy by harnessing the immune system’s inherent capability to particularly goal and destroy malignant cells. This customized therapeutic method entails genetically engineering a affected person’s personal T cells to specific artificial receptors—CARs—that acknowledge tumor-specific antigens independently of the key histocompatibility complicated (MHC). Central to this know-how is the CAR-T vector plasmid: an artificial DNA assemble that encodes the CAR protein and incorporates all the required regulatory components to make sure correct expression and performance inside T cells.
Understanding the structural parts of the CAR-T vector plasmid is crucial not just for optimizing therapeutic efficiency and security but additionally for advancing the sector of mobile immunotherapy. This text offers an in depth exploration of the CAR construction itself and the important vector components housed inside the plasmid that allow environment friendly CAR expression, choice, and performance.
1. The CAR Protein: Modular Construction and Operate
On the core of CAR-T remedy lies the chimeric antigen receptor, an artificial transmembrane protein that equips T cells with the power to acknowledge particular tumor-associated antigens and provoke cytotoxic immune responses. CARs are modular, typically composed of three key domains:
-
Ectodomain (extracellular antigen recognition)
-
Transmembrane area (membrane anchoring)
-
Endodomain (intracellular signaling and activation)
Every area is rigorously engineered to maximise T cell specificity, activation, and persistence.
1.1 Ectodomain: Concentrating on Tumor Antigens with Precision
The ectodomain protrudes from the T cell floor and mediates antigen recognition. It incorporates three vital subcomponents:
A) Sign Peptide
The sign peptide, typically derived from the CD8α chief sequence, is a brief amino acid sequence on the N-terminus that directs the nascent CAR protein to the endoplasmic reticulum. This facilitates correct folding, glycosylation, and transport to the cell floor. With out an efficient sign peptide, CAR proteins could fail to succeed in the membrane, rendering the remedy ineffective.
B) Antigen-Binding Area (scFv)
This area confers goal specificity. It’s generally a single-chain variable fragment (scFv), which is a fusion of the variable areas of a monoclonal antibody’s heavy (VH) and light-weight (VL) chains linked by a versatile peptide. The scFv binds to tumor antigens instantly on the cell floor, impartial of MHC presentation, permitting CAR-T cells to bypass tumor immune evasion mechanisms that downregulate MHC molecules.
The affinity and specificity of the scFv critically affect therapeutic outcomes. Whereas excessive affinity can enhance goal recognition, overly sturdy binding could trigger activation-induced cell dying or off-target toxicity. Moreover, the epitope location on the antigen and antigen density on tumor cells additionally have an effect on CAR-T efficacy. Notably, scFv domains are liable to aggregation, which may trigger tonic (antigen-independent) signaling resulting in untimely T cell exhaustion. Engineering steady, well-folded scFvs is thus an ongoing space of analysis.
C) Hinge or Spacer Area
The hinge area connects the scFv to the transmembrane area and offers structural flexibility, permitting the antigen-binding area to succeed in and interact goal epitopes that could be sterically hindered. The spacer size and composition considerably affect CAR perform.
Generally used spacers embody hinges from CD8α or CD28, or IgG Fc areas (e.g., IgG1 or IgG4). IgG-derived spacers should typically be mutated to forestall interplay with Fc gamma receptors (FcγRs) on innate immune cells, which may set off unintended immune responses and CAR-T cell clearance. Spacer size should be tailor-made to the particular antigen epitope; longer spacers facilitate entry to membrane-proximal epitopes however could enhance tonic signaling and activation-induced cell dying, whereas shorter spacers are extra appropriate for membrane-distal epitopes.
Apart from practical roles, spacers may also function epitopes for antibody-based CAR detection or purification methods, resembling incorporating Strep-tag sequences.
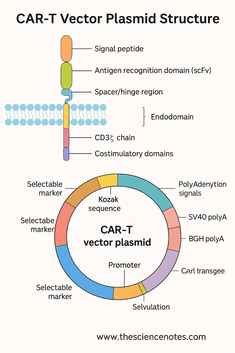
1.2 Transmembrane Area: Anchoring and Stabilization
The transmembrane area anchors the CAR within the T cell membrane and bodily connects the extracellular recognition area to the intracellular signaling modules. Regardless of its small measurement, this area impacts CAR expression ranges, receptor stability, and signaling.
Transmembrane domains are sometimes derived from native T cell proteins resembling:
-
CD3ζ (CD247)
-
CD4
-
CD8α
-
CD28
The selection of transmembrane area can affect how the CAR integrates with endogenous T-cell receptor (TCR) complexes. For example, CD3ζ transmembrane domains can promote CAR incorporation into native TCR complexes, doubtlessly enhancing activation however probably destabilizing receptor complexes. CD8α and CD28 transmembrane domains are likely to confer larger receptor stability and constant floor expression. Moreover, transmembrane domains can have an effect on cytokine launch profiles and tonic signaling, components carefully tied to security and efficacy.
1.3 Intracellular Signaling Domains: Initiating T Cell Activation
The intracellular endodomain interprets antigen recognition into T cell activation and cytotoxic response. It incorporates signaling motifs that set off proliferation, cytokine launch, and goal cell killing.
A) CD3ζ Chain
The CD3ζ chain is the first signaling area in all CAR designs. It incorporates three immunoreceptor tyrosine-based activation motifs (ITAMs) that, when phosphorylated upon antigen binding, provoke downstream signaling cascades important for T cell activation.
B) Costimulatory Domains
To reinforce T cell perform, persistence, and forestall exhaustion, CARs incorporate a number of costimulatory domains upstream of CD3ζ:
-
CD28: Promotes fast T cell enlargement and potent cytokine secretion however could result in shorter persistence.
-
4-1BB (CD137): Enhances T cell survival and reminiscence formation, contributing to sustained exercise.
-
Others: ICOS, CD27, OX40, and mixtures resembling MYD88 plus CD40 are below investigation for optimized signaling.
CAR generations are categorised based mostly on their intracellular area complexity:
-
First-generation CARs: CD3ζ alone.
-
Second-generation CARs: CD3ζ plus one costimulatory area (most FDA-approved CARs belong right here).
-
Third-generation CARs: CD3ζ plus two costimulatory domains for doubtlessly enhanced perform.
2. The CAR-T Vector Plasmid: Important Genetic Components
The CAR transgene is embedded inside a plasmid vector designed to maximise expression, stability, and choice functionality in T cells and through plasmid propagation.
2.1 Promoters: Driving CAR Expression
Environment friendly transcription of the CAR gene depends upon the selection of promoter controlling the expression cassette. Widespread promoters embody:
-
EF1α (Elongation Issue 1 alpha): A powerful, constitutive promoter lively in a broad vary of cell varieties together with T cells. It permits steady, high-level expression with out silencing.
-
CMV (Cytomegalovirus) quick early promoter: A viral promoter with strong early expression however may be silenced in sure main cells over time.
-
PGK (Phosphoglycerate kinase) promoter: A weaker, constitutive promoter used when average expression is desired to cut back tonic signaling or toxicity.
The promoter selection balances the necessity for ample CAR protein on the floor with minimizing dangers of overactivation or exhaustion.
2.2 Kozak Sequence: Optimizing Translation Initiation
Instantly upstream of the beginning codon (AUG), the Kozak consensus sequence (GCCGCCACC) enhances ribosome binding and initiates environment friendly translation of the CAR mRNA. Incorporation of an optimized Kozak sequence is customary observe to maximise protein yield.
2.3 Polyadenylation Alerts: Making certain mRNA Stability
Following the CAR coding sequence, polyadenylation (polyA) alerts promote correct transcription termination, mRNA stability, and nuclear export. Two generally used polyA sequences are:
-
SV40 late polyA: From simian virus 40, extensively utilized in mammalian expression vectors.
-
BGH polyA: Bovine progress hormone polyadenylation sequence, equally efficient at stabilizing transcripts.
Correct polyadenylation reduces degradation and enhances CAR protein manufacturing.
2.4 Selectable Markers and Reporter Genes
To establish and choose efficiently transfected or transduced T cells, plasmids typically embody markers resembling:
-
Antibiotic resistance genes (e.g., neomycin resistance): Permit for antibiotic choice throughout cell tradition.
-
Fluorescent proteins (e.g., EGFP, mCherry): Allow visualization and sorting through movement cytometry.
-
Twin markers: Combining antibiotic resistance and fluorescence for versatile choice.
These components are sometimes managed by separate promoters and don’t intrude with CAR expression.
2.5 Origin of Replication (ori)
For plasmid propagation in micro organism, an origin of replication resembling pUC ori is included. This enables high-copy replication in E. coli, facilitating large-scale plasmid manufacturing vital for scientific and analysis purposes.
2.6 Bacterial Choice Marker
The ampicillin resistance gene (Amp^R) is used for antibiotic choice in bacterial tradition, guaranteeing solely micro organism harboring the plasmid survive.
3. FDA-Authorised CAR-T Merchandise: Actual-World Vector Examples
A number of CAR-T cell therapies have obtained FDA approval, every utilizing totally different however basically comparable vector designs.
| Commerce Identify | Generic Identify | Firm | Approval Date | Goal Antigen | Indications |
|---|---|---|---|---|---|
| KYMRIAH™ | Tisagenlecleucel | Novartis | Aug 2017 | CD19 | Relapsed/Refractory B-ALL |
| YESCARTA™ | Axicabtagene ciloleucel | Gilead/Kite | Oct 2017 | CD19 | Relapsed/Refractory DLBCL |
| TECARTUS™ | Brexucabtagene autoleucel | Gilead/Kite | Jul 2020 | CD19 | Relapsed/Refractory Mantle Cell Lymphoma |
| BREYANZI® | Lisocabtagene maraleucel | Bristol Myers Squibb | Feb 2021 | CD19 | Relapsed/Refractory Massive B-cell Lymphoma |
| ABECMA® | Idecabtagene vicleucel | Bristol Myers Squibb | Mar 2021 | BCMA | Relapsed/Refractory A number of Myeloma |
| CARVYKTI® | Ciltacabtagene autoleucel | Janssen | Feb 2022 | BCMA | Relapsed/Refractory A number of Myeloma |
These merchandise exemplify how modular CAR and vector designs are tailored to focus on totally different antigens and ailments whereas sustaining a core plasmid spine structure.
https://www.most cancers.gov/about-cancer/therapy/analysis/car-t-cells
4. Concluding Remarks: The Way forward for CAR-T Vector Design
The CAR-T vector plasmid is way over a easy DNA supply automobile; it’s a complicated, finely tuned genetic device important for profitable CAR-T cell remedy. Every part—from the scFv’s affinity and spacer size, by the selection of transmembrane and signaling domains, to promoter energy and choice markers—impacts the therapeutic final result.
Whereas first- and second-generation CARs have achieved exceptional scientific successes, ongoing challenges embody antigen escape, T cell exhaustion, and toxicity. These points spotlight the necessity for improved vector designs, together with inducible promoters, novel costimulatory domains, optimized scFvs, and security switches.
As CAR-T therapies broaden past hematological malignancies into stable tumors and different ailments, vector plasmids might want to evolve with superior regulatory components and finely tuned CAR architectures to satisfy new challenges. Enhanced high-throughput screening and computational design instruments will speed up this improvement, in the end enhancing efficacy, security, and accessibility for sufferers worldwide.